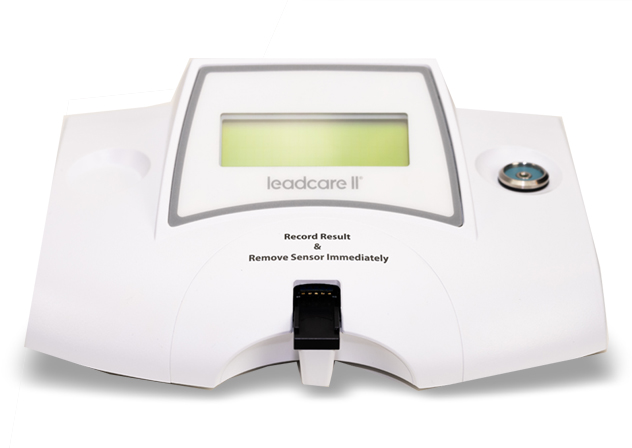
LeadCare II
LeadCare II is the only CLIA-waived, point-of-care blood lead testing system. The system includes the LeadCare II Blood Lead Analyzer and the LeadCare II Test Kit and is designed for the quantitative measurement of lead in fresh whole blood.
LeadCare II is manufactured by Meridian Bioscience, a fully integrated life science company that develops, manufactures, markets and distributes a broad range of innovative diagnostic products. It is headquartered in Cincinnati, Ohio.
The LeadCare II relies on electrochemistry (Anodic Stripping Voltammetry or ASV) and a unique sensor to detect lead in whole blood. Most lead is carried within red blood cells. When a sample of whole blood is mixed with treatment reagent, the red blood cells are lysed and the lead becomes available for detection. When a test is run, the analyzer applies an electrical potential that causes the lead to collect on the sensor. After 3 minutes, the analyzer measures the amount of lead on the sensor and displays the result in micrograms per deciliter (µg/dl).
No, the LeadCare II Test Kit is specific for the analysis of lead only.
LeadCare II is the only CLIA-waived, point-of-care blood lead testing system. The test can be performed on site, requires only two drops of blood, and provides a quantitative blood lead result in 3 minutes.
In contrast, traditional lead testing methods require sending the test to an outside lab. With traditional tests, a blood sample (capillary or venous) is collected in the doctor’s office or clinic and sent out for testing at a reference lab, or the child is sent to another laboratory to have their blood collected and then sent out for analysis. Traditional testing methods require physicians to wait days to weeks for results, and parents must be called back for follow up. With LeadCare II, testing is performed while the parent and child are in the office, providing the perfect opportunity for education and intervention all in one visit.
Lead analysis is performed in three simple steps:
The user collects 50 µl (approximately 2 drops) of whole blood from the patient’s fingertip, using a capillary tube provided in the test kit.
The user mixes the patient sample with prepackaged treatment reagent. A chemical reaction liberates the free lead from the red blood cells.
Using a transfer dropper supplied in the kit, the user places a drop of the test mixture on a disposable sensor that has been placed in the analyzer. Lead analysis begins automatically and the result is displayed 3 minutes later.
Yes, blood lead testing is reimbursable using CPT code 83655 (‘QW’ modifier may be required). Reimbursement varies by state and insurance plan. For information to assist you regarding reimbursement in your state contact Reimbursement@magellandx.com.
Federal law requires blood lead testing of all Medicaid enrolled children at ages 12 and 24 months and any 3- to 6-year olds that have not been tested previously. A lead test is required for enrollment in Head Start Programs.
Each state has its own regulations governing testing for children who are not enrolled in Medicaid. For help as you evaluate the current testing requirements in your state, contact the Product Support Team at (800) 275-0102 or email us at LeadCareSupport@magellandx.com.
The LeadCare II Blood Lead Testing System is approved as a CLIA-waived test. This means that any employee of an office/laboratory operating under a CLIA Certificate of Waiver (or higher) can perform the test as long as they follow the manufacturer’s instructions.
Some states have additional licensing requirements. Check with your state’s laboratory licensing department or contact the Product Support Team at (800) 275-0102 or email us at LeadCareSupport@magellandx.com for more information about your state’s specific licensing requirements.
Per the Clinical Laboratory Improvement Amendments of 1988 (CLIA ‘88), waived tests are categorized as simple laboratory examinations and procedures that have an insignificant risk of an erroneous result. The Food and Drug Administration (FDA) determines the criteria for tests as being simple with a low risk of error and is responsible for approving manufacturer’s applications for test system waiver.
Obtaining a CLIA Certificate of Waiver from your state agency involves an application and a modest annual fee. For instructions on how to obtain a CLIA Certificate, visit: Getting Started.
Yes, the office/lab performing the test is responsible for reporting all blood lead results to the appropriate state agency. Every state has different requirements – for help in understanding your state’s reporting requirements, contact the Product Support Team at (800) 275-0102 or email us at LeadCareSupport@magellandx.com.
We also offer a complimentary software program for download, to assist you in electronic reporting.
About Lead Poisoning
Lead poisoning occurs when too much lead builds up in the body. Exposure to even small amounts can cause health and learning problems in children – most notably long-term consequences in academic achievement and behavior problems. However, most children with elevated lead levels don’t have obvious symptoms, and problems associated with learning and behavior may not appear until they reach school-age.
Lead exposure occurs when a person inhales lead-containing fumes or dust or swallows something that contains lead. Lead-based paint and lead-contaminated dust are the main sources of exposure for U.S. children.1
Although lead-based paints were banned for use in U.S. housing in 1978, most homes built before then contain some lead-based paint, which releases into the air and into dust when it deteriorates.
The Centers for Disease Control and Prevention (CDC) estimates that 24 million housing units have deteriorated lead paint and elevated levels of lead-contaminated house dust – about 1 in every 3 U.S. housing units may have lead paint hazards.
The CDC notes other sources of lead include tap water from corroded pipes, candy from Mexico, some traditional medicines used by East Indian, Indian, Middle Eastern, West Asian, and Hispanic cultures, some toys and toy jewelry, and artificial turf. For more information, see CDC’s Prevention Tips for Sources of Lead.
Toddlers and young children tend to explore with their hands and frequently put them in their mouths. As young children crawl on the floor and reach windows, railings, and walls, they may inhale dust from peeling and chipping lead-based paint or ingest paint chips.
Furthermore, children under six years of age are biologically more sensitive to lead, due to rapid brain and organ development, when the body readily takes up lead—mistaking it for calcium. Therefore, children who are low in calcium or have iron-deficient anemia may have an ever greater uptake of lead.
Federal law requires that all Medicaid enrolled children be tested at 12 and 24 months and 3 to 6 years if they have not previously been tested.
Beyond this federal requirement, state mandates and guidelines vary. For information about your state’s specific testing requirements, contact the Product Support Team at (800) 275-0102 or email us at LeadCareSupport@magellandx.com.
Children with elevated levels of lead in their bodies often do not seem sick. Some initial symptoms are very general – including headache, stomach pain, lack of appetite or energy, constipation, agitation, clumsiness, and sleepiness – which all could have other causes. In extreme cases, a child may have vomiting, stupor and convulsions and require emergency attention.
A simple blood test using the LeadCare II at your local public health clinic or family doctor can reveal if your child has too much lead in their blood. This test involves taking a few drops of blood from your child’s fingertip. Children who test positive initially should have a confirmatory test using a venous sample and performed by a clinical laboratory. Adults who think they may have been exposed to too much lead at work, through a hobby, or a home renovation project should also be tested.
Your doctor is the best person to help explain what to do if your child has elevated blood lead levels. The best way to lower an elevated blood lead level is to prevent continued exposure to lead, and to make sure your child is receiving good nutrition, as having enough calcium and iron is important to help slow the body’s uptake of lead. For very high blood lead levels, a doctor may prescribe medicine that combines with lead so the body can get rid of it more easily. It is recommended that all children with elevated levels receive ongoing monitoring.
Preventing exposure to lead involves both temporary measures to create barriers between the child and the sources of lead exposure, as well as measures to remove the lead sources permanently. See below, “What can I do to prevent lead poisoning?” for more details.
No level of lead in the blood can be defined as safe. Evidence has been building regarding health and developmental problems in children with blood levels below 10 μg/dL, which traditionally was regarded as the level of concern. An advisory panel of the CDC now recommends that children with blood levels as low as 5 μg/dL be identified as having elevated levels and be monitored. Even such low levels may harm a child’s cardiovascular, immunological, and endocrine health, with consequences for that individual and for overall public health. While the effects of lead appear to be irreversible, eliminating lead exposure can reduce further damage.
Yes, adults can become lead poisoned, most frequently from the workplace and hobbies. Lead in adults can affect the central nervous and gastrointestinal systems and can cause chronic kidney disease as well as other health problems.
According to the CDC, prenatal lead exposure has known influences on maternal health, infant birth and neurodevelopment outcomes. Because bone lead stores are activated during pregnancy for women with prior lead exposure, lead released into maternal blood and breast milk can adversely affect the fetus or newborn. Talk to your doctor about lead and blood lead testing if you think you may be at risk.
The best way to prevent lead poisoning among young children is to remove the source of lead. To prevent exposure, especially in homes built before 1978, the CDC recommends1:
- Contact your local health department about testing paint and dust from your home.
- Make sure a child does not have access to peeling paint or chewable surfaces painted with lead-based paint.
- Create barriers between children and lead sources until clean-up is complete.
- Regularly wet mop floors, damp sponge walls and all horizontal surfaces and vacuum with a high efficiency particulate air vacuum (HEPA vac). Cleaning is a temporary solution until the lead can be completely removed.
- Pregnant women and children should stay away from pre-1978 homes if renovation is underway.
- Regularly wash children’s hands, especially before eating.
- Regularly wash children’s toys.
- Prevent children from playing in bare soil, opting instead for sandboxes or for planting grass. This is especially true of areas that are within one block of a major highway or busy street, as lead that was once in gasoline may have accumulated in the soil.
- Give your children foods that are high in calcium and iron, such as meat, beans, spinach, and low-fat dairy products. This can help reduce the amount of lead absorbed by the body.
Don’t see your frequently asked question here? Contact us
*These FAQs are intended as an introduction for those interested in learning more about lead poisoning. The information should not be used as a substitute for the care and advice of your physician.